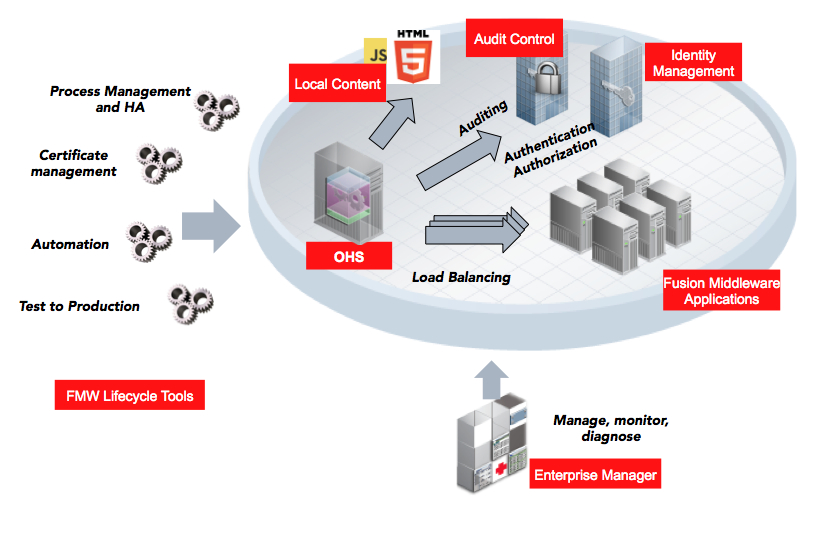
|
Content Serving / Reverse Proxy
Cloud Deployment / Virtual Server
Support
Thousands of sites / application domains
served from a single web server instance.
Each virtual server can have its own
configuration files, IP addresses, port,
document root, preferences, log files, and
more.
Protection From Common Threats
Built-in ModSecurity module provides the ability
to configure rules to introspect and
protect applications from common attacks
including SQL/Command injection, Cross
Site Scripting vulnerabilities and other
vulnerabilities.
FastCGI Support
Efficient way to serve dynamic content web
pages within OHS by using scripting
languages such as PHP or Python, without
incurring a significant performance penalty.
Integrated Reverse Proxy
Built-in proxy modules provide generic proxy
support as well as optimized support for
WebLogic Server, allowing OHS to act as
the HTTP end-point for HTTP origin servers
including WebLogic Server.
|
Administration
/ Monitoring
Server Administration
Leverages WebLogic 12c administration
interfaces to provide a simple, consistent
and distributed administration model for
administering Oracle HTTP Server, Oracle
WebLogic Server and the rest of the Fusion
Middleware Stack.
For more information, please refer to Understanding
the OHS Administration Model section.
Monitoring
Integration with Oracle Enterprise Manager
allows customers to monitor HTTP traffic by
using the Oracle Enterprise Management console.
Robust Migration Tool
Integrated migration tools make it easy
to migrate existing Oracle HTTP Server 11g
deployments to Oracle HTTP Server 12c. |